German Stocks Spike on Passage of Draft Law
Synbiotic SE (SBX)
Yesterday, the German federal cabinet officially passed the draft law which would seek to implement Pillar 1 of the country’s ambitious adult-use cannabis legalisation strategy.
The passage of the ‘law on the controlled handling of cannabis and on changing other regulations’, known as ‘CanG’, marks a major step forward for the country’s campaign, though the bill will still need to be passed by the German Bundestag and Bundesrat.
However, according to the Ministry of Health, the bill does not require approval in the state chamber and it is expected to come into force by the end of the year.
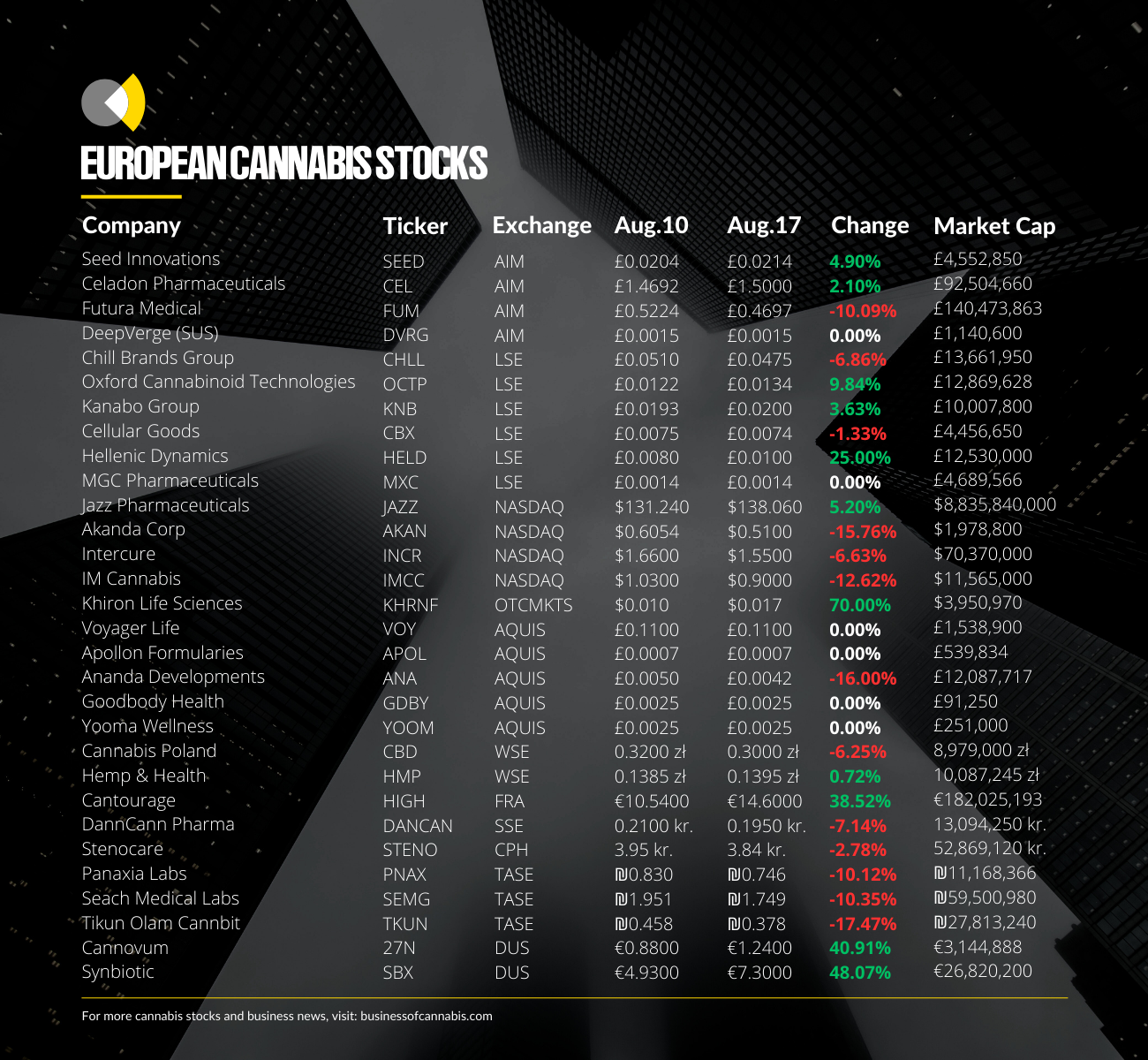
The news has already sent stocks of all the German cannabis operators tracked by Business of Cannabis jumping by double digits, with some international operators seeing significant upticks.
Cantourage (HIGH)
German cannabis group SynBiotic SE experienced the strongest spike this week, seeing its stock price jump by nearly 50% to levels not seen since April.
This positive investor sentiment also saw Frankfurt-listed Cantourage’s stock jump by 38.5%.
Cannovum Cannabis AG, which is now solely focused on the German adult-use cannabis space, also saw its stock increase by over 40% on the Dusseldorf Exchange.
Business of Cannabis will be delving deeper into this development, and what it means for the German cannabis project and the businesses hoping to capitalise on it, in the coming day
Jazz Pharmaceuticals
The pharmaceutical giant and manufacturer of Epidyolex published its Q2 2023 results this week, seeing its shares jump by over 6% as it raised its financial guidance for the full year.
In the three months to June 30, 2023, Jazz reported total revenues of $957.3m, representing a 3% year-on-year increase and beating consensus estimates, while earnings per share almost tripled from $0.55 to $1.52.
It attributed this upside to sales of three main compounds, Xywav, Rylaze and its market-leading medical cannabis formulation Epidyolex, which together account for ‘66% of total revenues’.
The company’s chairman Bruce Cozadd said: “We’ve achieved another quarter of double-digit, year-over-year growth of Epidiolex, as we unleash its blockbuster potential through in-person engagement, compelling data and ongoing educational efforts.”
Epidyolex saw net product sales increase by 15% year-on-year during the quarter to $202.2m, as its ‘global launch continued to gain momentum’.
This included the start of a Phase 3 trial of the drug for Dravet syndrome, Lennox-Gastaut syndrome and tuberous sclerosis complex in Japan.
Meanwhile, its other cannabis-based compound Sativex saw sales decline 32% year-on-year, dropping from $4.1m in Q2 2022 to $2.8m this year.
Jazz now expects revenue for the full year to come between $3.725bn and $3.875bn, up from estimates in May of between $3.625bn and $3.875bn, thanks to an expected rise in its ‘neuroscience’ division sales.
Analysts at MorningStar said that while they expect the landmark $7.2bn acquisition of Epidyolex manufacturer GW Pharmaceuticals to further dilute returns, Epidyolex has ‘blockbuster potential by 2025 thanks to its strong efficacy and robust launches in the US and Europe’.
Furthermore, it said it expected upcoming ‘patent losses over the next several years for some of Jazz’s other drugs’ to further dilute earnings.
Ananda Developments
Aquis-listed Ananda Developments announced this week that the NHS has now pledged £300,000 to its upcoming endometriosis randomised controlled trial (RCT).
In March this year, Ananda acquired MRX Global, alongside the rights to a CBD formulation that has just been approved for two Phase 2 clinical trials in the UK with £1.55 million of grant funding.
Now, Ananda says it has received a further ‘non-dilutive’ cash injection from the Chief Scientist Office (CSO), the part of the Scottish Government Health Directorate responsible for funding research.
“Getting NHS funding for this trial using MRX1 is a clear statement of the importance of endometriosis as a public health issue and we believe clearly demonstrates the interest in the use of cannabidiol as a potential treatment for endometriosis and other complex chronic inflammatory pain conditions by the UK’s public health bodies,” Ananda’s CEO Melissa Sturgess said.
“The chronic, complex inflammatory pain market was recently estimated to be worth at least £5 billion per annum in the UK alone.”
The upcoming endometriosis RCT will see 100 participants take either Ananda’s patent pending MRX1 cannabidiol oil or a matching placebo.