Cannovum Cannabis AG
German listed cannabis operator Cannovum Cannabis AG announced this week that it now plans to ‘outsource its existing successful medical business’.
Business of Cannabis recently reported that Cannovum had chosen to pivot its strategy following the roll-back of Germany’s plans to launch a fully commercial adult-use cannabis market.
At the time, the company said that while there was a renewed focus on its medical arm, it was adamant that it is not only ‘not disregarding recreational’, but that it is also ‘ideally positioned’ to capitalise on the opportunities as they stand.
Now, as part of its efforts to be ‘fully focused on the upcoming legalisation of cannabis in Germany’, the company has announced a separation of its operations.
Founder Pia Marten will now lead the ‘outsourced’ medical cannabis offshoot ‘Cannovum Health eG’.
As part of a ‘management buy-out’ Ms Marten will leave the board of Cannovum Cannabis AG, handing the reins over to the former CMO, and new CEO, Klaus Madzia.
Ms Marten said: “Cannovum Health eG will focus on the already regulated medical cannabis market. Through new capital brought into the company, we will focus on accelerating the growth of Cannovum Health eG. Especially with the focus on extract distribution we aim for sustainable profitability. I wish Cannovum Cannabis AG under the leadership of Klaus Madzia much success and thank you for the good cooperation.”
Mr Madzia added: “The legalisation of cannabis in Germany is imminent. We see a unique opportunity in this billion-dollar market and are concentrating our forces. The cultivation alliance for Germany founded by Cannovum Cannabis is already the pacesetter for legalisation.”
Pia Marten: “Cannovum Health eG will focus on the already regulated medical cannabis market. Through new capital brought into the company, we will focus on accelerating the growth of Cannovum Health eG. Especially with the focus on extract distribution we aim for sustainable profitability. I wish Cannovum Cannabis AG under the leadership of Klaus Madzia much success and thank you for the good cooperation.”
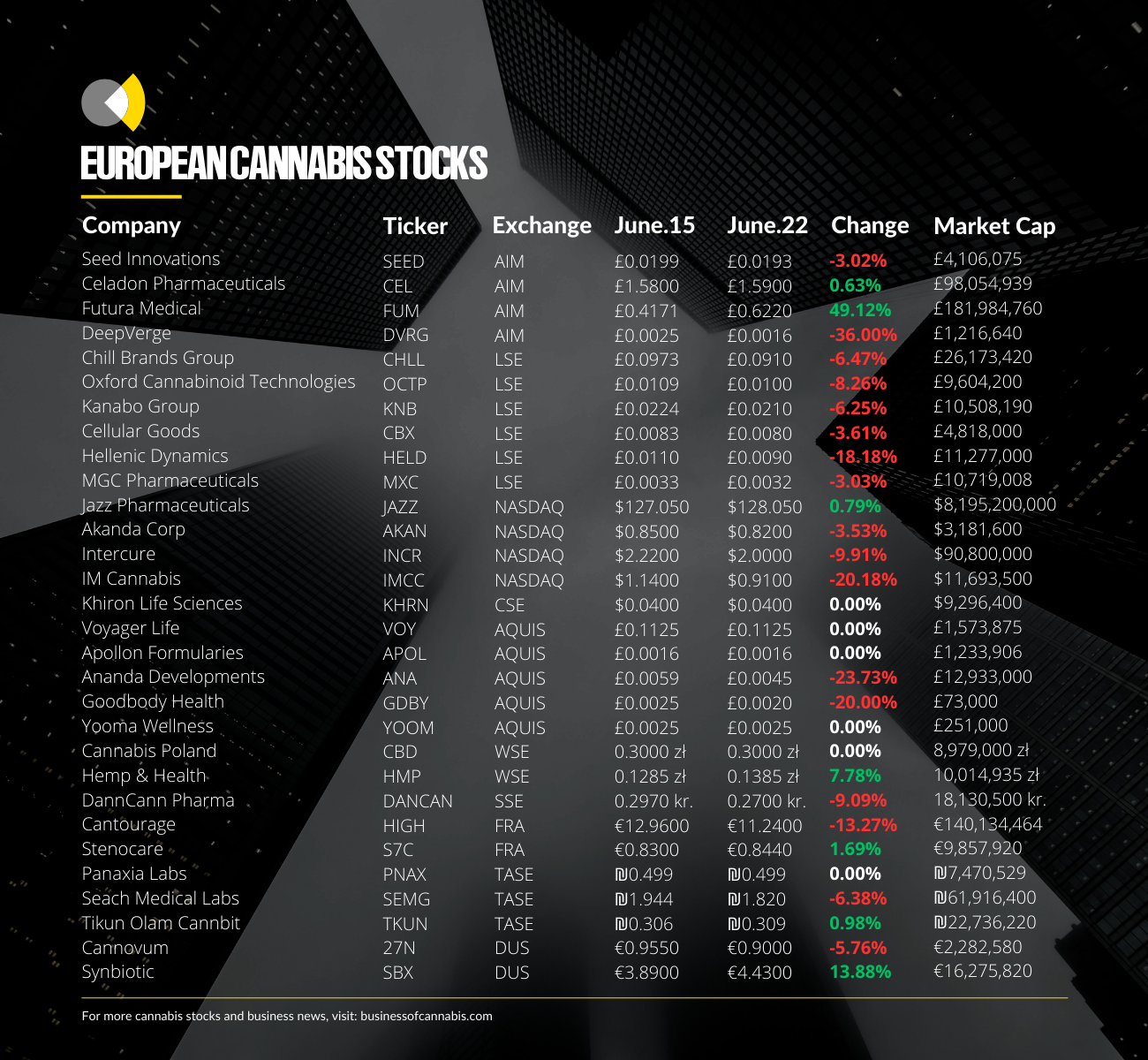
Futura Medical
Futura Medical’s stock price has spiked by nearly 50% this week after the company announced the receipt of FDA approval for its Eroxon gel.
Eroxon is an erectile dysfunction treatment that uses Futura’s proprietary DermaSys skin delivery method.
This technology is also being developed by the company to deliver CBD through the skin via a product called CBD 100, which the company calls a ‘uniquely stable cannabidiol formulation, effective penetration for enhanced therapeutic benefits with fast, effective and long-lasting action.’
After becoming the first non-prescription erectile dysfunction treatment available in the UK in April 2023, launching in high street retailer Boots and online stores throughout the country, it is now set to be the first in the US also.
Futura announced on June 12 that ‘the US Food and Drug Administration (FDA) has granted over the counter sale (OTC) Marketing Authorisation for MED3000’, which trades as Eroxon.
This means the product can now be legally marketed and sold in the US, Belgium and the UK, with ‘commercial agreements’ in Switzerland, South Korea, Latin America and the Middle East set to see further rollouts throughout 2023.
Alongside the FDA approval, Futura announced that it had raised a further £4.37m via a warrant exercise from Lombard Odier Asset Management.
In light of this, 10,937,500 ordinary shares were admitted to AIM on June 16.
“Support from all our shareholders provides the company with continued cash security from which to grow and look to the future with confidence,” Futura’s CEO James Barder said.
“I welcome today’s announcement and the support from Lombard Odier, as these funds provide Futura Medical with good visibility into 2025 as the Company prepares for the next chapter of growth with anticipated launches, and the scale up of commercialisation of MED3000”.
Stenocare
Danish medical cannabis operator Stenocare published its Q1 2023 financial figures, reporting ‘progress’ despite ‘modest sales’.
In the three months to March 31 2023, Stenocare recorded net sales of DKK808,000, down from DKK896,000 a year earlier.
On a yearly basis however, the company reported sales of DKK4.5m throughout 2022, more than double sales of DKK1.9m in 2021.
Stenocare’s EBITDA losses were reduced slightly year-on-year, falling from DKK3.4m in Q1 2022 to DKK3.1m in the same period this year.
The news comes just a week after Stenocare completed a rights issue with a total subscription rate of around 127%, providing the company with gross proceeds before transaction related costs of DKK10.7m.
CEO Thomas Skovlund Schnegelsberg said of the recent results: “Stenocare’s brand and market footprint is growing, but despite being seen as an emerging market leader, we are still in the early phase of our scale-up.
“In a market like ours – that is emerging ‘in front of us’ – we have to accept and expect that our development is not linear, when it comes to sales. It is a pharma market – not a consumer market. In essence, our business is about so much more than simply driving monthly sales in existing markets and infrastructures.”