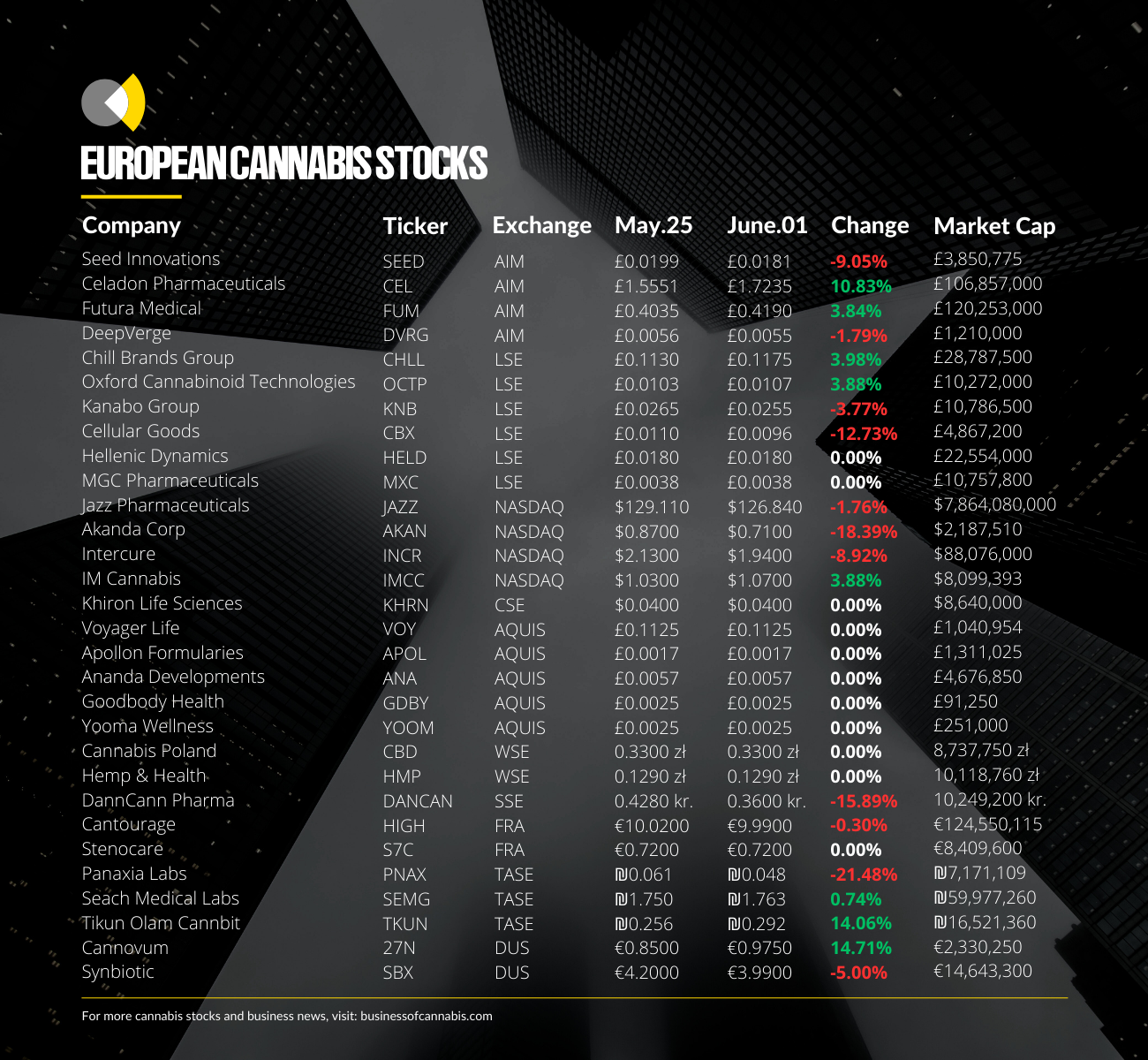
Akanda
Akanda has seen its stock drop by nearly 20% this week as news broke that a short-term loan agreement, announced just a month ago, had now been scrapped.
The international medical cannabis company saw its stock fall from around US$0.9 to US$0.7 over the past week, meaning the company’s stock is now once again in breach of NASDAQ’s minimum bid price requirements.
In March, the company announced that it had regained compliance with the listing rules, which require the company’s share price to remain above $1, following a share consolidation.
The fall in share price comes as Akanda informed investors that its ‘short-term loan’ deal, which was set to see Veridia Canada provide $500k, was no longer going ahead.
According to a press release published earlier this week, the parties ‘have mutually agreed to terminate the loan agreement’, which Interim CEO Katie Field said at the time provided Akanda with ‘the flexibility needed to continue the implementation of our strategic plan while continuing to evaluate longer-term financing options’.
Though little detail was provided as to the reasons behind this, Akanda said that it has ‘returned all funds provided under the terms of the loan agreement and has now further obligations owing to’ Veridia.
Tikun Olam Cannbit
Israeli cannabis operator Tikun Olam Cannbit also scrapped a potentially significant deal this week, announcing that its proposed merger with BOL Pharma was no longer going ahead.
The pair signed a ‘memorandum of understanding’ on April 3, which would have seen the companies combine to create a single new entity which would be traded on the Tel Aviv Stock Exchange.
Weeks later, on May 21, Tikun Olam announced that negotiations with BOL had now ended ‘without proceeding to sign a merger agreement’.
While, once again, little detail was provided as to the reasoning behind this development, some reports suggested that it was due to Tikun Olam considering activities outside of cannabis.
According to Israeli Cannabis Magazine,Tikun Olam announced in mid-May that it was ‘examining the feasibility of entering additional fields’.
It assured investors that this would be done ‘simultaneously with the cannabis activity’, as opposed to exiting the cannabis industry for good like a number of its peers.
Despite this, any efforts to invest or merge with a company outside of cannabis would effectively thwart its tie-up with BOL.
Doubts were raised at the time as to how serious its intentions to enter other markets were, and whether this was simply a tactic to leverage its position in negotiations with BOL.
News of the scrapped merger has sent Tikun Olam’s stock down a further 14% this week, seeing it continue to trade at all-time lows.
Zelira Therapeutics
Outside of Europe, Australian biopharma company Zelira Therapeutics saw its ASX-listed stock more than triple this week.
The company, which calls itself ‘a global leader in the development of clinically validated cannabis medicines’, announced this week that its proprietary drug ‘outperformed’ a market-leading compound produced by pharmaceutical behemoth Pfizer.
Its cannabinoid-based compound ZLT-L-007 was pitted head-to-head against Pfizer’s Lyrica in its effectiveness at treating diabetic nerve pain.
According to the ‘groundbreaking study’, conducted in the US, the topline results demonstrate Zelira’s compound outperformed Lyrica’s in achieving a ‘significant reduction’ in NRS pain scores, in some cases four times as much as its rival.
For context, Lyrica currently generates around US$5bn in revenues every year for Pfizer, a figure which has been distributed widely among investors, seeing Zelira’s share price jump from A$0.93 to A$3.05 in a matter of days.
The study’s Principal Investigator Dr Bryan Doner said: “Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica level of pain relief.
“I am particularly pleased that the topline data reveals no reported serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout.”
The company, which already sells cannabinoid-based products in the UK, US and Australia, says the results ‘provide confidence to evaluate the further progression of ZLT-L-007 into formal FDA clinical trials’.