AFTER a lively first day of sessions focused on the future of the cannabis industry, the second day of Cannabis Europa London 2023 opened with a look back at the industry’s very beginnings.
Regulatory consultant Esther Farkash opened the first session in the Barbican’s theatre with a tribute to Raphael Mechoulam, the ‘Father of Cannabis Research,’ who died this March at the age of 92.
Industry pioneers
She recalled how Professor Mechoulam, who studied cannabis and the endocannabinoid system for 60 years, began his prolific career in cannabis by befriending officers at his local police station, who gave him 5 kg of cannabis from the evidence room to begin his work.
“This is why we understand THC and CBD today,” Ms Farkash explained.
After determining that there must be a ‘built-in system in our body that reacts so strongly to these compounds’, he went on to work for 30 years on researching the endocannabinoid system, publishing over 700 scientific papers in the world’s most prestigious scientific publications, and writing the guidelines for how patients are treated by medical cannabis.
“I would like to finish with a sentence he used to mention time and again: ‘Modulating the endocannabinoid system may have therapeutic potential to treat all forms of diseases’. Thank you all for continuing his work and vision.”
In a fitting tribute, the day ended with a speech from another cannabis industry pioneer, Professor David Nutt, who gave the audience an exclusive insight into the groundbreaking Project Twenty21 initiative conducted by Drug Science.
Prof. Nutt walked through the ‘truly remarkable’ findings of the three-year study of around 3,000 patients into the effects of regular cannabis use on a range of specific conditions and on the patients’ general wellbeing.
Within three months of regular usage, patients, around 50% who suffered from chronic pain, and a further 41% who suffered from psychiatric disorders such as anxiety, saw a marked improvement in their baseline reported general health metrics.
Reports of feelings of depression decreased markedly, ‘easily as much as antidepressants’, while sleep quality improvement was also ‘highly significant, and patients’ self-reported metrics for anxiety almost halved.
“That is massive. I doubt if any trial on anxiety has had an effect size that big.”
Chronic pain and PTSD patients also recorded a marked improvement, news that will be ‘very, very satisfying for patients’.
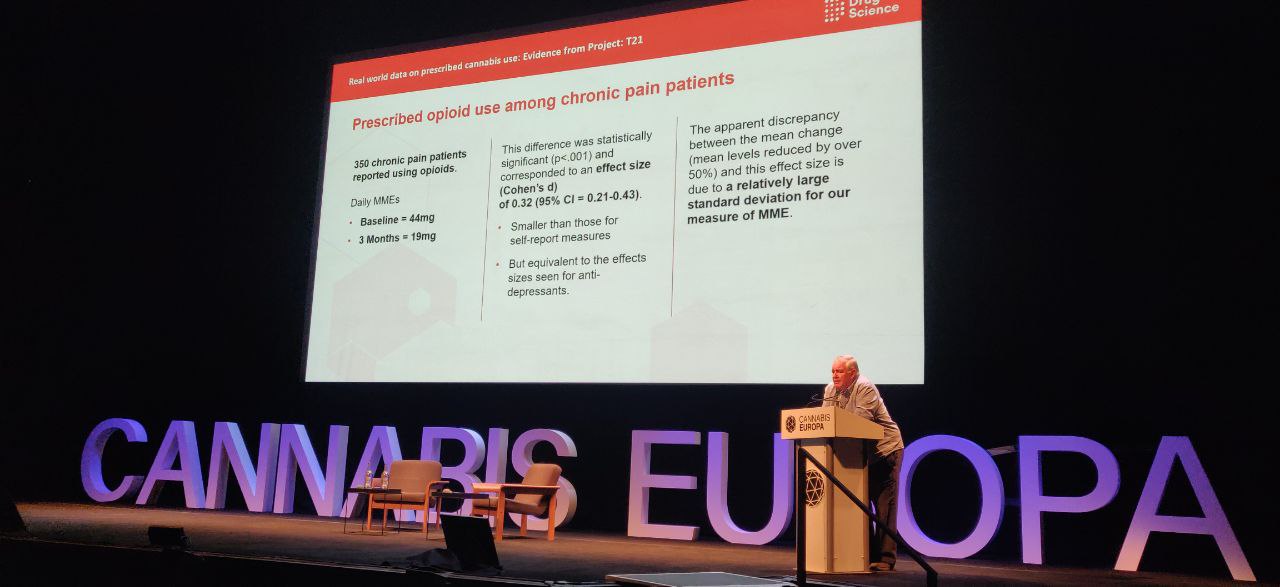
Furthermore, patients who were also using opiates to treat their disorders saw a significant reduction in usage, with the baseline dosage of Milligram Morphine Equivalent (MME) dropping from 44 mg to 19 mg.
“We didn’t direct any alterations in medication. I want to emphasise that these are patients that are choosing not to use opiates. And this is untargeted. It’s almost certain we could do better if we made the effort.
“I think now this is evidence enough to warrant proper systematic assessment because the evidence we’ve provided for tolerability and efficacy is unquestionably outstanding.”
Expanding research
This was not the only groundbreaking cannabis research conducted by Drug Science on the agenda during day two, with a panel of experts deep diving into cannabis’ potential to treat long COVID, which is estimated to affect around 2m people in the UK as of January 2023.
Drug Science’s Senior Research Officer Hannah Thurgur began by explaining that the six-month phase-two feasibility study looked primarily to establish the safety of treating these patients with a CBD-dominant compound, but on a secondary level sought to explore the effects of cannabis on their quality of life, fatigue, depression and anxiety.
She explained while the sample size was ‘smaller than we would have liked’ at 12 participants, it showed promise in the deterioration of symptoms even after coming off the medication.
Founder and Medical Director of the London Resilience Clinic Dr Dani Gordon said: “At least you have set the groundwork for someone else to come in and do the next phase of this study. These things are so bureaucratic, so this is a really important piece of work.”
Dr Gordon went on to explain that she found treating chronic fatigue with cannabis through an integrative medicine approach was effective in helping patients engage with their treatment programmes.
“If we can get them a little bit more energy, then they can engage with the rest of the programme. Cannabis is a catalyst for them to engage with the next steps of their programme. Cannabis is the only thing I have found that can do this.”
This session also opened a debate, which extended throughout the day, regarding the type of research being conducted into medical cannabis, its cost, and its value to patients and the medical industry.
Ms Thurgur suggested that despite some speakers dismissing real-world evidence in favour of RCTs, there was ‘real value in trying to see how more reflective representative populations of patients respond to different types of cannabis-based products, as well as being able to have this more personalised approach to that diversity’.

With a trio of individual interviews into the changing role of the pharmaceutical industry in the European cannabis industry, this topic was explored in far greater detail.
Jorja Emerson Centre’s Managing Director Robin Emerson argued that the industry needed to ‘do a lot more hard work’ to provide the clinical evidence that we require to move the industry forward in the UK.
Brains Bioceuticals’ Terry O’Regan largely agreed with this, stating that the industry was ‘restricted because no one’s doing those clinical studies’, meaning it was starting from the ground up.
“It breaks my heart when I see these failed cannabis studies, and I wonder whether it really didn’t work or they just didn’t do the due diligence in dosage. These studies are giving a negative impression of this industry, and the last thing we want is for it to be relegated to sort of homoeopathic medicine.”
He suggested that getting the pharmaceutical industry involved was vital to access funding for this ‘expensive clinical research’, but, in order to do that, the industry needed to ‘hold ourselves to the highest standards’.
Finally, Curaleaf’s Chief Medical Officer Dr Mikael Sodergren pushed back against this, highlighting the fact that real-world evidence was now becoming more widely accepted in the medical community.
“The MHRA, the EMA and the FDA have all released press releases stating that they are very happy to use real-world evidence to accelerate drug development. Now, real-world evidence will never replace clinical trials – that won’t happen – but it certainly has a role to make the process faster and cheaper.
“We have this feedback loop which is not available to pharma in traditional drug development, and that is real-world evidence. This kind of recording programme is something that can be uniquely applied to medical cannabis.”
Lessons to be learned
Despite the growing momentum of cannabis research across the continent, the parallel momentum of adult-use markets threatens to undermine the former’s progress.
Avicanna’s CEO Aras Azadian told a panel exploring the growing ‘identity crisis’ surrounding the adult-use and medical markets that there was a ‘lot to learn from Canadian mistakes’.
“We always pushed for medical to be prioritised – we saw the opposite. The industry was so focused on getting rich from rec, which never happened, that the medical market was neglected.
“Because we knew rec was coming, we had medical companies that were really just recreational companies. The medical community was misled to believe there was going to be investment in trials and then no one saw that…The medical community has been completely upset by what happened.”

He argued that after the recreational market came in, physicians were no longer engaged with the industry or willing to be educated, with many assuming would-be patients would simply turn to the recreational market.
Regarding Germany’s decision to ‘step back from rec’, he said he thought the news was ‘fantastic for patients’.
The University of London’s Dr Kojo Koram emphasised this point, stating: “I think there is a sense among some policy makers that the issues with medical cannabis could be solved by a recreational market, allowing patients to access that medicine through those pathways. This runs the risk of undermining the legitimacy of both markets.”
In a subsequent session focused on the reasons to be optimistic about Germany’s recent developments, Alephsana’s Boris Moschowitz explained that he believed Germany could still be a leading example for cannabis regulation.
Unlike countries, including Luxembourg, Spain and Portugal, where legislation is ‘not executed well’, he suggested in Germany there was at least a ‘true will to execute what they put on paper’.
“If we can put that energy into all of the other European countries together in one direction, I’m quite confident we’ll succeed.”
Tickets for Cannabis Europa 2024 are now on general sale. You can book online by clicking here.























